
PAXLOVID tablets
Standard Dose Pack
The standard dose is recommended for patients with no known renal impairment (eGFR >90 mL/min) OR mild renal impairment (eGFR ≥60 to <90 mL/min):
- The standard dose of PAXLOVID is 2 nirmatrelvir 150 mg tablets and 1 ritonavir 100 mg tablet taken together in the morning and at bedtime, with or without food, for 5 days
- PAXLOVID should be administered at approximately the same time each day for 5 days
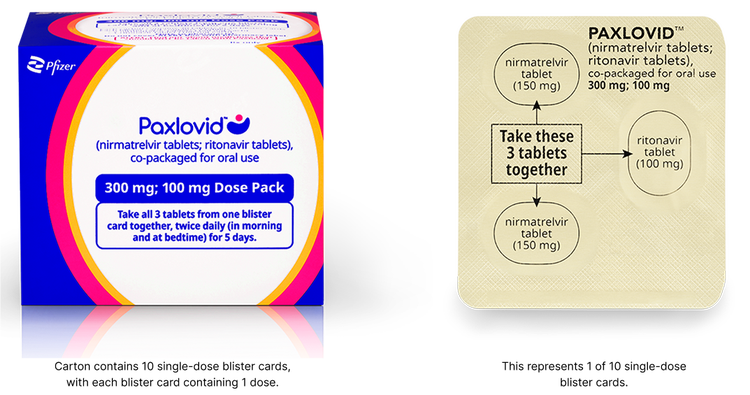

Patients should complete the entire course of treatment as prescribed, with twice daily dosing
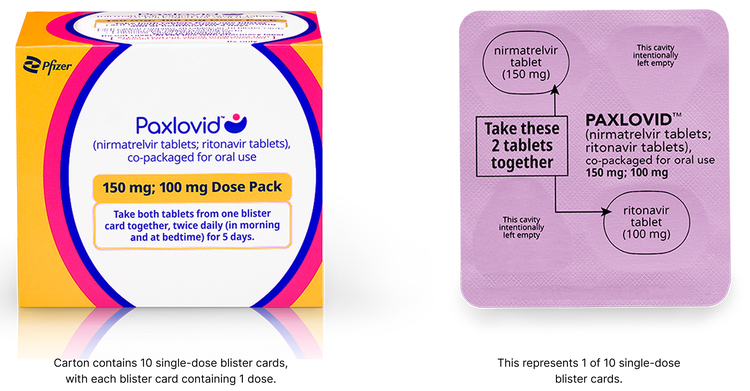
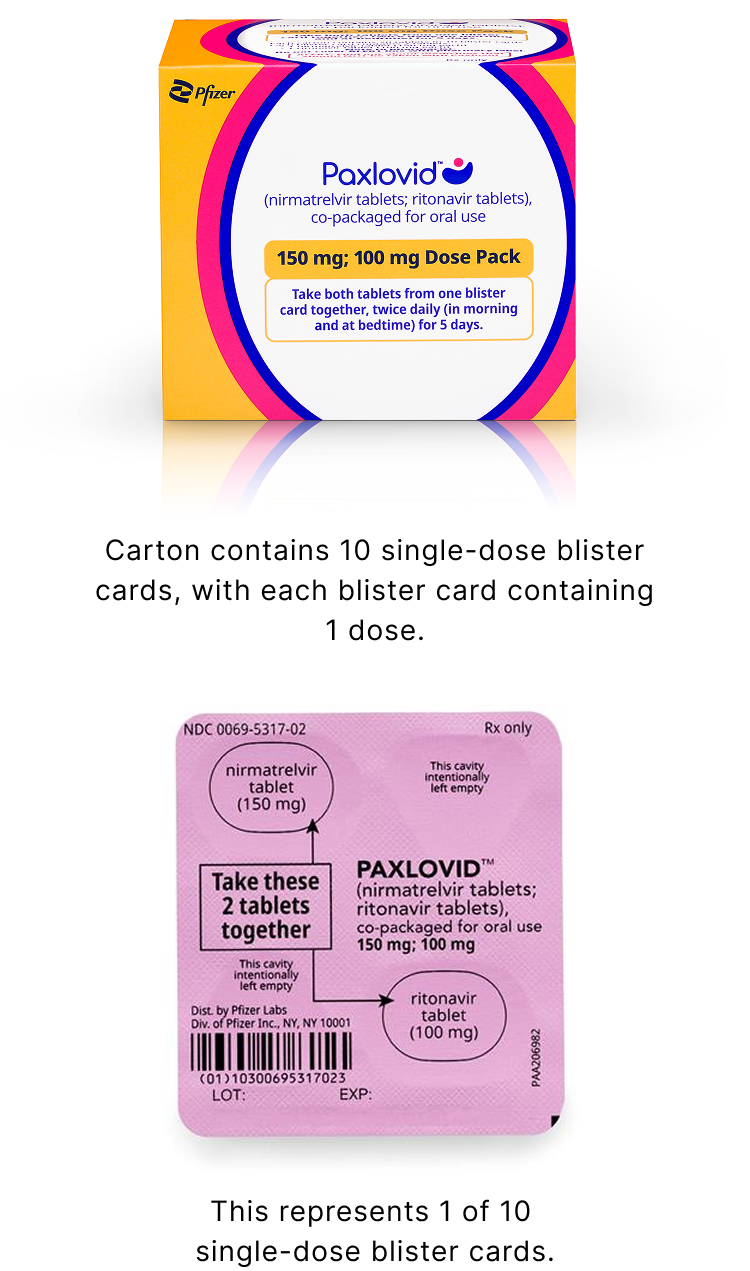
Reduced Dose Pack: for patients with moderate renal impairment
The reduced dose is recommended for patients with eGFR ≥30 to <60 mL/min:
- 1 nirmatrelvir 150 mg tablet and 1 ritonavir 100 mg tablet taken together in the morning and at bedtime, with or without food, for 5 days
- PAXLOVID should be administered at approximately the same time each day for 5 days
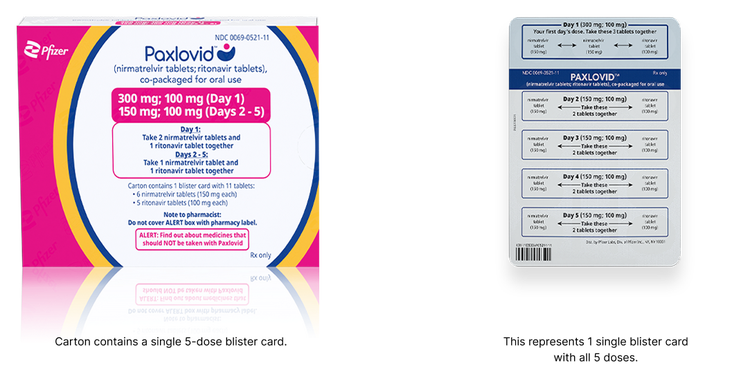
Severe Renal Impairment Dose Pack
Supply available Q2 2025
The severe renal impairment dose is recommended for patients with eGFR <30 mL/min, including those requiring hemodialysis:
- Day 1: 2 nirmatrelvir 150 mg tablets (300 mg) and 1 ritonavir 100 mg tablet taken together
- Days 2 to 5: 1 nirmatrelvir 150 mg tablet and 1 ritonavir 100 mg tablet taken together
- PAXLOVID should be administered at approximately the same time each day for 5 days
On days of hemodialysis, the PAXLOVID dose should be administered after hemodialysis.

Hepatic Impairment
No dosage adjustment is needed in patients with mild (Child-Pugh Class A) or moderate (Child-Pugh Class B) hepatic impairment. No pharmacokinetic or safety data are available regarding the use of nirmatrelvir or ritonavir in participants with severe (Child-Pugh Class C) hepatic impairment; therefore, PAXLOVID is not recommended for use in patients with severe hepatic impairment.

Important administration information
- Nirmatrelvir must be coadministered with ritonavir. Failure to correctly coadminister nirmatrelvir with ritonavir may result in plasma levels of nirmatrelvir that are insufficient to achieve the desired therapeutic effect
- Prescriptions should specify the numeric dose of each active ingredient in PAXLOVID. Healthcare providers should counsel patients about reduced dosing instructions
- The 5-day treatment course of PAXLOVID should be initiated as soon as possible after a COVID-19 diagnosis and within 5 days of symptom onset
- Alert the patient of the importance of completing the full 5-day treatment course and of continuing isolation in accordance with public health recommendations to maximize viral clearance and minimize transmission of SARS-CoV-2
- If the patient misses a dose of PAXLOVID within 8 hours of the time it is usually taken, the patient should take it as soon as possible and resume the normal dosing schedule. If the patient misses a dose by more than 8 hours, the patient should not take the missed dose and instead take the next dose at the regularly scheduled time. The patient should not double the dose to make up for a missed dose

Dose adjustments
- No dosage adjustment is recommended in patients with mild renal impairment (eGFR ≥60 to <90 mL/min)
- No dosage adjustment is required when coadministered with other products for HIV containing ritonavir or cobicistat
- Patients on ritonavir- or cobicistat-containing HIV or HCV regimens should continue their treatment as indicated
What would you like to learn more about?
Drug Interactions
View boxed warning, contraindicated drugs, and other potentially significant drug interactions.
Efficacy
See the efficacy data from the phase 2/3 EPIC-HR trial of adult participants with a laboratory-confirmed diagnosis of SARS-CoV-2 infection at high risk for progression to severe disease.
Safety
Find out about the safety data from clinical studies and post-authorization experience and how to report an adverse event.
COVID-19=coronavirus disease 2019; eGFR=estimated glomerular filtration rate; HCV=hepatitis C virus; HIV=human immunodeficiency virus; SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
Reference:
- Fact Sheet for Healthcare Providers: Emergency Use Authorization for PAXLOVID™. Pfizer Inc.; 2025.

